Al3+ Ion Electron Configuration
3 pts Which has a larger radius Al. The electron configuration of the aluminum shows that it has three unpaired electrons in the last shell3s 1 3p x 1 3p y 1.

Electron Configuration For Aluminium Al
1s2 2s2 2p3 d.

. What is the electron configuration of the Al3 ion. Kr5s2 4d10 5p6 6s1 d. The Mg atom is in Group 2A of the periodic table and will lose 2 electrons to form Mg 2 ion.
Electron Configuration for Fe Fe2 and Fe3 Iron and Iron Ions In writing the electron configuration for Iron the first two electrons will go in the 1s orbital. Electronic configuration of Al 3. Normally a neutral atom of aluminum in a grounded state would have 13.
What is the electron configuration of Al 3. The p orbital can hold up to six electrons. 4 pts Write the full electron configuration for the Al3ion.
3 pts Name a neutral atom which shares the same electron configuration as Al3 ion. So in the 3 oxidation state aluminium attain the noble gas electronic configuration. QUESTION 15 Which best.
Oct 29 2019 - In this video we will write the electron configuration for Al 3 the Aluminum ion. What is the ground-state electron configuration of the ion. The stable ion of aluminum is Al 3 which means it has three fewer electrons.
See answer 1 Best Answer. The Ca 2 ion is therefore isoelectronic with the. Chemistry questions and answers.
It is formed by losing 3. The electronic configuration of Al 3 is 1 s 2 2 s 2 2 p 6. We know in general that the electron configuration of Aluminum Al is 1s 2 2s 2 2p 6 3s 2 3p 1.
1s2 2s2 2p6 Which of the following is the correct noble gas electron configuration for an ion of barium. Now in the Al 3 ion the positive charge means Aluminum loses three electrons. Well also look at why Aluminum forms a 3 ion and how the electron confi.
Therefore the valency of aluminum is 3. To predict the actual C-O-C bond angle in βglycoside bond one has to draw the actual conformation. The aluminum atom has shed its outer shell of 3s2 3p1 and has an electron configuration equal to that of Neon or 1s2.
The p orbital can hold up to six electrons. In this video we will write the electron configuration for Al 3 the Aluminum ion. Well also look at why Aluminum forms a 3 ion and how the electron configuration for Al3 is.
Well put six in the 2p orbital and then put the next two electrons in the 3s. Kr5s2 4d10 5p6 6s2 b. 1s2 2s2 2p1 e.
1s2 2s2 2p6 3s2 3p1 c. 1s2 2s2 2p6 3s2 3p4 b. When the Aluminium Al atom loses three electrons it forms Al 3 ion.
Its electronic configuration is has 10 electrons and 13 protons. When a Ca atom loses both of its valence electrons the result is a cation with 18 electrons a 2 charge and an electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6. The next six electrons will go in the 2p orbital.
For example calcium is a group 2 element whose neutral atoms have 20 electrons and a ground-state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2. In this case the valency of. The electron configuration of an aluminum ion Al3 is 1s2 2s2 2p6.
The nex six electrons will go in the 2p orbital. Therefore the Aluminium electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 1. Since the 3s if now full well move to the 3p where well place the remaining electron.
Given that during a lab experiment vanillin was added to a Erlenmeyer flask. Chemistry Standard XI Suggest Corrections 0 Similar questions Q. View the full answer.
Since 1s can only hold two electrons the next 2 electrons for Iron go in the 2s orbital. B At the start of lab Anthony adds vanillin to his Erlenmeyer flask then adds sodium hydroxide. After the electron configuration the last shell of the aluminum atom has three electrons.
For example sodium has an electron configuration of Ne3s 1Sodium will lose one electron from the 3s subshell to form Na 1The configuration for the Na 1 ion is Ne. Electron configurations for cations and anions are determined from the electron configuration of the parent atom. What is the ground-state electron configuration of the Al3 ion.
How many valence electrons does aluminum ionAl 3 have.

Electronic Configuration Of Al Al3 Youtube

Ba 2 Electron Configuration Barium Ion Youtube
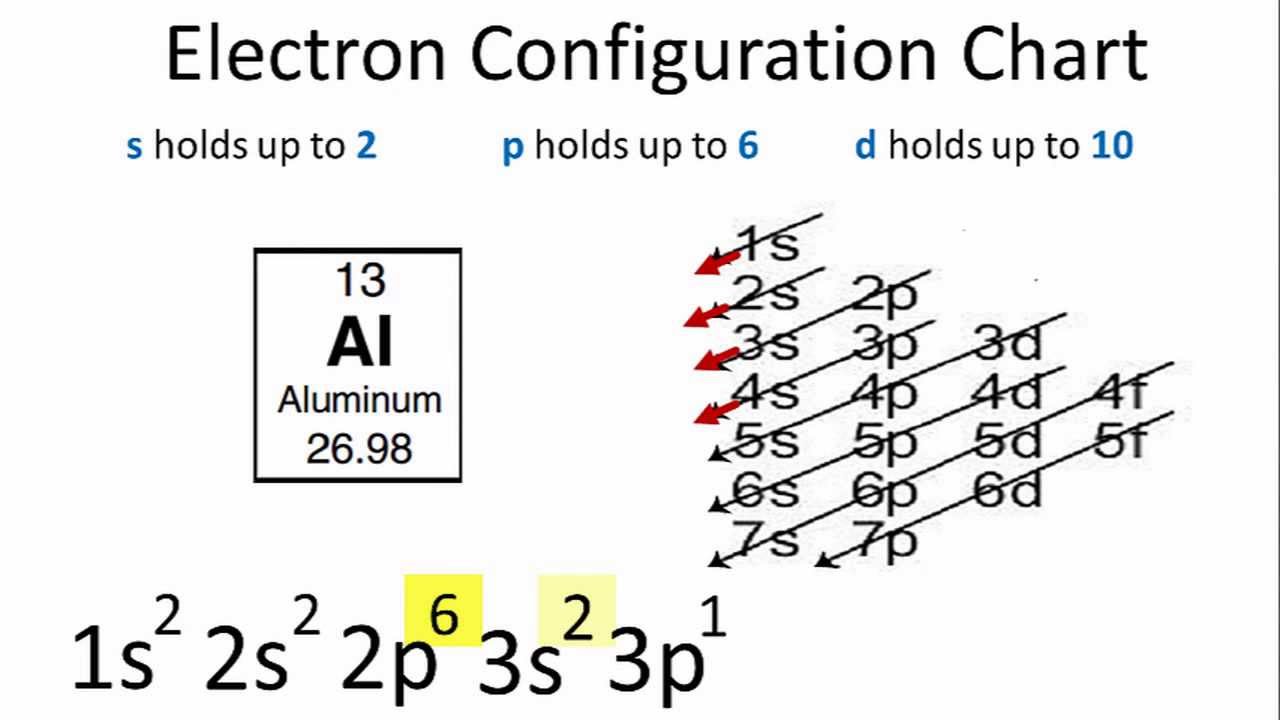
Electron Configuration For Aluminium Al

Al 3 Electron Configuration Aluminum Ion Youtube

Al 3 Electron Configuration Aluminum Ion Electron Configuration Electrons Configuration

Aluminum Orbital Diagram Electron Configuration And Valence Electrons
0 Response to "Al3+ Ion Electron Configuration"
Post a Comment